App-based solution
Simplify SAP EH&S - secure, user-friendly and company-wide
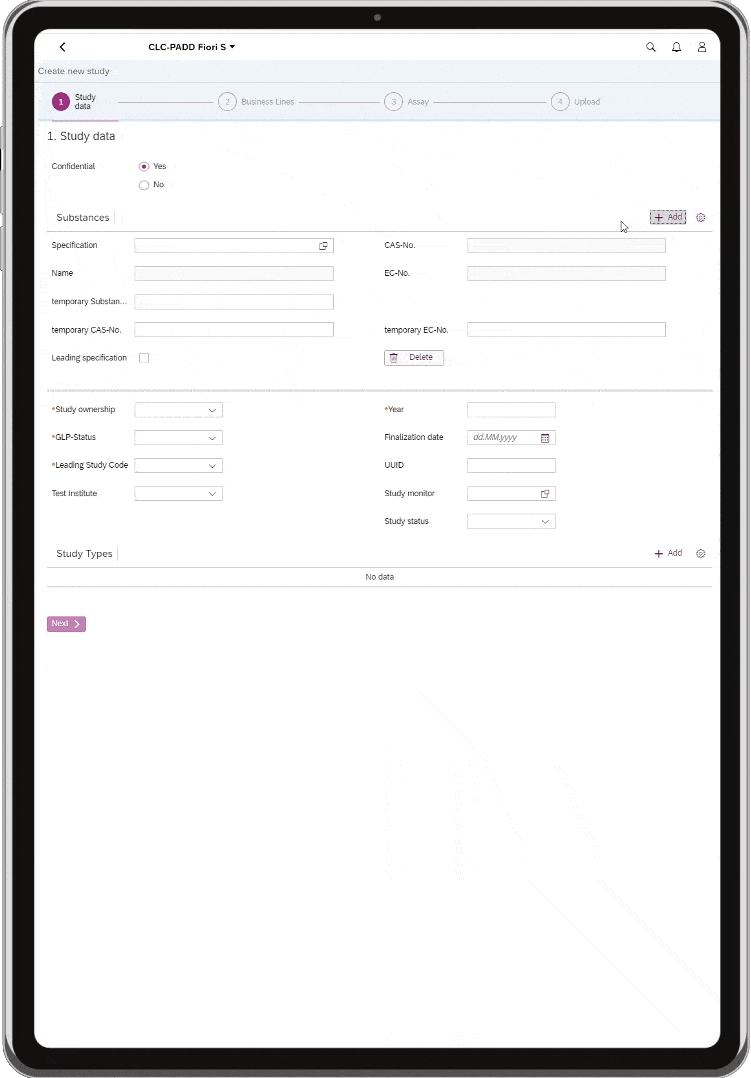
App-based solution for efficient management of study data and study reports in SAP
How efficient management of study data and reports in SAP ensures quality and saves time and money. Based on the ESIMS (Evonik Substance Information Management System) project, Dr. Kai Blumbach, Head of Hazard & Risk Management at Evonik Operations GmbH (Nutrition & Care Division) and Cedric Schonard, expert at CLC xinteg GmbH, describe the path from isolated landscapes to a secure, user-friendly and company-wide solution.
Today, modern companies work with intuitive business apps that support users in database maintenance and data management. On the way there, it is necessary to convert historically grown and technologically outdated isolated solutions into future-proof systems.

CLC & EVONIK interview
Dr. Blumbach, what was the initial situation before you, together with CLC xinteg GmbH, set the course for the ESIMS (Evonik Substance Information Management System)?
Evonik is committed to making life better, day after day. Of course, we also follow this motto within the Group. ESIMS should be a future-proof SAP-integrated solution platform in which all safety-relevant data such as study reports, results and metadata on substances can be stored and managed. And in a direct way. The existing isolated island landscapes with all their disadvantages should be abolished and replaced by intelligent business apps. It should be possible to manage all safety-relevant data on substances across divisional boundaries.
What did the project steps look like in detail? And what results were you able to achieve, Mr. Schonard?
All relevant data from various legacy databases was integrated and migrated into one platform so that ESIMS is now connected to the SAP EH&S system as a modern application. With the help of the CLC-PADD® solution suite, intelligent business apps were installed in the EVONIK SAP system, which enable simple and efficient data management.
This enables interdisciplinary collaboration between scientists and toxicologists, such as creating and centrally managing studies securely, efficiently and quickly. Comprehensive authorization protection secures access for employees of individual divisions. This ensures compliance and security at all times.
Dr. Blumbach, what do you think of the new solution?
First of all, a big compliment to everyone involved in the project. Together, we have created an outstanding solution in just two months. ESIMS is now a clear, company-wide system into which data from various sources has been migrated and can be maintained in a central location using the same system. More specifically, the connection via CLC-PADD allows us to® to SAP EH&S, all data can be processed quickly and without loss via a direct interface. This ensures our high quality standards and saves a lot of time. Our IT department now only manages one tool, the SAP add-on CLC-PADD®which reduces support costs as much as possible.
Sensitive, i.e. confidential, data such as studies, most of which also represent a high financial value for Evonik, used to be stored in some

"self-made" tools. Today, they are located in a security environment with SAP standard and cannot be accessed unprotected via external access points.
At a glance and across divisional boundaries, you can immediately see which studies were conducted with which OECD guideline. Products or substances can be easily exchanged between divisions: We simply change their
organizational assignment and the substance "migrates" to the new support. In the past, this would have meant lengthy handover discussions and slow document transfer. Now, a field is simply changed and the substance is assigned a new owner.
Dr. Blumbach, what other positive insights have you gained from the joint project with CLC xinteg?
To put it in a nutshell: island landscapes and silo thinking are history for Evonik. The trusting collaboration has brought us all much closer together across divisional boundaries. The result is a shared view of how we store the data and metadata associated with a study in a future-proof way. In addition, we are now following a smart path for further processing our data. Last but not least, the project was also a complete success from an economic point of view. We are very satisfied with the new, user-friendly ESIMS, which is secure in every respect, and are already working on the next projects with CLC xinteg, such as the replacement of an existing Oracle database for the registration of substances in accordance with the REACH regulation.

Mr. Schonard, where is the journey heading in the course of further cooperation with Evonik?
Now that our SAP add-on CLC-PADD® has been installed, further application scenarios and requirements can be implemented relatively quickly and within budget. In line with the principle of "one tool - any process", we are currently implementing strategic applications that ensure and facilitate compliance with and tracking of the numerous legal requirements worldwide, such as REACH-UK, TSCA or KKDIK. With the introduction of a product file, we are also creating a standardized source of information for product-related data and documents.